Fatty Acids Definition in Biology: These are acids occurring in natural triglycerides and are monocarboxylic acids. Fatty acids are composed of long hydrocarbon chains terminated by carboxylic acid groups. Fatty acids are basically the primary derivative of lipids. Chain length from 4 to usually 24C atoms. They contain even number of C atoms majority of fatty acids are those containing 16 and 18 C atoms. Fatty Acid Structure Described Below.
Fatty Acid Structure
Fatty Acid Formula

Examples of Fatty Acids:
Types of Fatty Acids
- Saturated Fatty acids.
- Unsaturated Fatty acids.
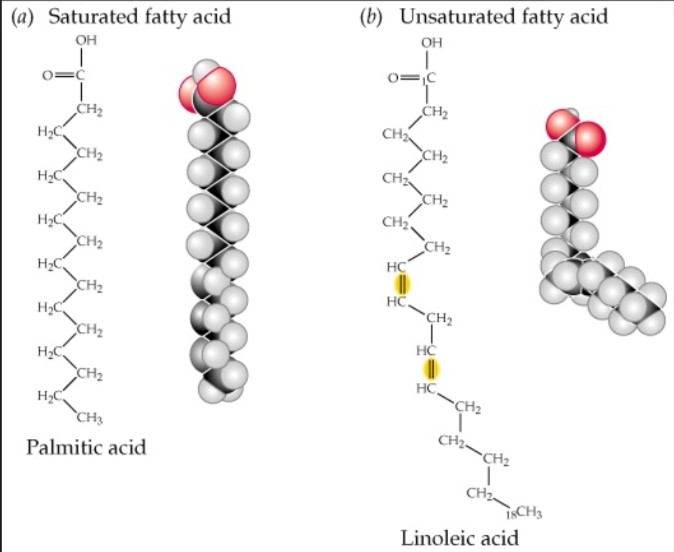
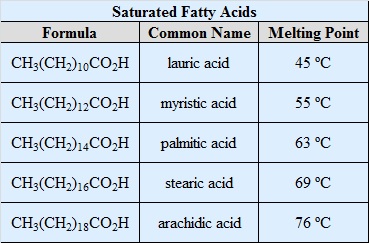
Saturated Fatty Acids Definition
- These fatty acids have a single C-C bond. They are solid at room temperature. Saturated fatty acids usually contain 12-22 carbon atoms. For e.g Ghee, Butter, Palmito-oleic acid etc.
Examples of Saturated Fatty Acid

These formulas have two symbols first show carbon atoms while other shows double bonds
Unsaturated Fatty acids Definitions:
- These fatty acid have the Double C-C bond. Unsaturated fatty acids are LIQUID at room temperature.
- If one double bond present in fatty acid it is called Monosaturated or Monoenoic fatty acids.
- If more than one double bond present in fatty acid it is called Polysaturated or Polyenoic fatty acid. For e.g. vegetable oil.
Examples of Unsaturated Fatty Acid:
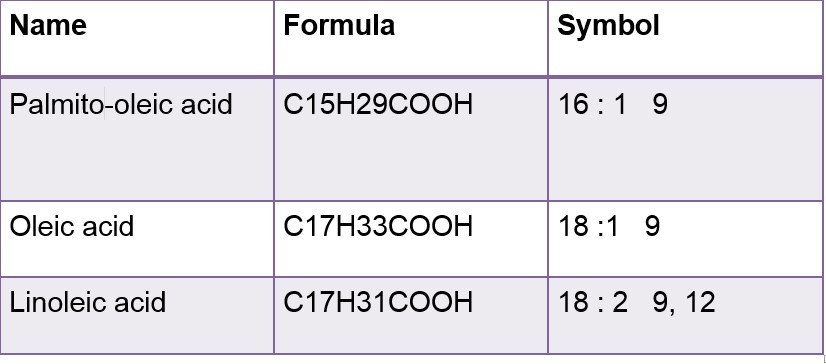
In its symbols form First no. represent C numbers and Second no. represent the double bond. Greek letter delta( ) signifies double bond.
Physical Properties of Fatty Acid:
- Saturated fatty acids are solid at room temperature.
- Unsaturated fatty acids are liquid at room temperature.
- The long chain length of fatty acid has a high melting point than short-chain fatty acids.
- The solubility of fatty acids decrease due to increase in no. of the methylene group
- Presence of double bond increases the solubility of fatty acids.
- Acetic acid is completely miscible with water because it contains only 1 methyl group. Palmito-oleic acid is more soluble than palmitic acid.
- Fatty acids form salts with alkali and alkaline earth metal.
- Salts of sodium, potassium, calcium, and magnesium are formed when fatty acids react with these salts.
- Fats have the specific gravity less than 1 and, also lower than water, therefore, they float on water.
- The melting point of fats depends upon their constituent fatty acids. Greater the amount of unsaturated fatty acids in the fat molecule, lower will be the melting point.
- Freshly prepared fats are colorless, odorless and tasteless. Any color or taste is due to association with other foreign substances. Example: The yellow color of Butter or is due to the presence of plant pigments carotene and xanthophyll.
Chemical Properties of Fatty Acids:
Following are the properties of fatty acids:
- Hydrolysis
- Saponification
- Reactions due to unsaturation
- Saponification number
- Rancidity
Hydrolysis:
This can be accomplished by heating fats with water at high temperature and pressure. The hydrolysis of ingested fats is efficiently accomplished by enzyme Lipase present in pancreatic juice.
Saponification:
By boiling with strong alkanes such as NaOH, the fats are readily decomposed into Glycerol and salts of constituent fatty acids (Soap).
Reactions due to Unsaturation
The double bonds of unsaturated fatty acids in fats undergo the reaction such as:
- Hydrogenation
- Halogenation
- Oxidation
Hydrogenation:
Hydrogenation of liquid vegetable fats is done commercially to obtain solidified fats like “Banaspati” and Margarine.
Halogenation:
Neutral fats containing unsaturated fatty acids have the ability to add halogens. The degree of halogenation is an index of the unsaturated fatty acid content of fats.
Iodine Number:
“The number of grams of iodine which will be absorbed by 100 grams of fat is termed its iodine number”.More unsaturated fatty acids have higher iodine number value than more saturated fatty acids.
Saponification Number:
“It is the number of Milligrams of KOH required to neutralized the fatty acids liberated from 1 gram of fat.”Short chain fatty acids have a higher saponification number. Long chain fatty acids have lower saponification number. Example: Butterfats has a relatively higher saponification number, as it is rich in short-chain fatty acids.
Rancidity:
Many fats develop an unpleasant odor and taste when they are allowed to stand in contact with air at room temperature.
You May Also Like: Functions of Lipids | Definition | Classification | Examples
The Process of Rancidity:
Rancidity is due to two different processes:
- Oxidative Rancidity
- Hydrolytic Rancidity
Oxidative Rancidity
- Oxidation of fat molecules gives rise to some short chain aldehydes and ketones which have taste and odor.
- The oxygen of the air is necessary for the occurrence of this type of rancidity.
Prevention from Oxidative Rancidity :
Hydrolytic Rancidity
This rancidity is due to the slow hydrolysis of fats. In case of fats like Butter results in the liberation of short chain fatty acids. Which have odor and taste? Hydrolysis of fats may be hastened by Bacterial contaminants which produce enzyme lipase. Rancidity also results in a loss of certain essential dietary constituents such as vitamins A and E, Carotenes and linoleic acid.

